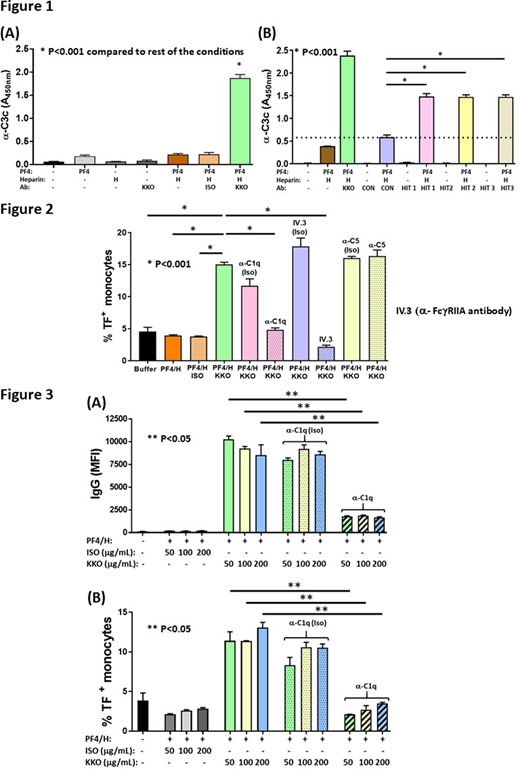
Heparin induced thrombocytopenia (HIT) is a prothrombotic disorder mediated by ultra-large immune complexes (ULICs) containing IgG antibodies bound to multivalent complexes of platelet factor 4 (PF4) and heparin (H). HIT ULICs activate cellular FcγIIA receptors that initiate diverse cellular effector functions including neutrophil degranulation and monocyte expression of tissue factor (TF). Previous studies have shown that HIT ULICs also potently activate complement through the classical pathway (Cines et al., 1980). Whether complement activation contributes to FcγRIIA-dependent prothrombotic pathways has not been addressed in detail. In studies that follow, we describe: 1) robust complement activation by HIT ULICs in plasma and whole blood (WB), 2) cell-surface deposition of complement and IgG triggered by HIT ULICs, 3) complement-dependent neutrophil degranulation and monocyte TF expression, 4) efficacy of proximal, but not terminal, pathway inhibition in regulating monocyte TF expression, and 5) deposition of complement in thrombi formed in "HIT mice" that generate ULICs containing KKO, a HIT-like monoclonal antibody (Arepally et al., 2000).
Consistent with prior studies showing involvement of the classical pathway in HIT (Cines et al., 1980), we observed that binding of C1q induced marked enlargement of HIT ULICs in buffer assessed by dynamic light scattering as well as in plasma using confocal microscopy (data not shown). To assess complement activation by HIT ULICs, we incubated WB and plasma with PF4 (25 µg/mL) ± heparin (1 U/mL) in the presence of KKO (or isotype, "ISO"; 50 µg/mL) or HIT IgG (or control IgG, "CON"; 500 µg/mL) and measured C3c with a capture immunoassay as previously described (Khandelwal et al., 2018). KKO (Figure 1A) or HIT ULICs (n=3; HIT1-3, Figure 1B), showed robust generation of C3c in the presence of PF4/heparin, but not antigens alone or with control IgG (ISO/CON). Complement activation by HIT ULICs leads to downstream generation of C5a and formation of sC5b-9 (data not shown). Pre-incubation of plasma or WB with a variety of classical pathway inhibitors, including a C1r inhibitor derived from Borrelia burgdorferi (BBK 32), C1 esterase inhibitor (Berinert, CSL Behring) and anti-C1q antibody (α-C1q Ab; Annexon Biosciences) inhibited C3c generation by KKO ULICs (p <0.001), whereas inhibitors of the alternative pathway (anti-properdin antibody) or C5 inhibitor (α-C5 Ab; Eculizumab, Alexion Pharmaceuticals) did not (data not shown). Incubation of WB with KKO or HIT ULICs, but not ISO or CON IgG, markedly increased deposition of C3 and IgG on neutrophils, monocytes and B cells (data not shown) and lead to cell activation assessed by neutrophil degranulation (MMP9 release) and monocyte TF expression (data not shown). To examine the contribution of complement activation in monocyte TF expression, WB was pre-incubated with α-C1q, α-C5 or IV.3 (a monoclonal antibody to FcγRIIA) or isotype controls prior to addition of HIT ULICs. As shown in Figure 2, the classical pathway inhibitor, α-C1q Ab markedly diminished TF expression (about 70% reduction; p<0.001 vPF4/H/ KKO), as did IV.3 (about 85% reduction; p<0.001 vPF4/H/ KKO) but not α-C5 Ab or ISO antibodies, demonstrating: 1) FcγRIIA independent mechanism of monocyte TF expression and 2) a requirement for proximal rather than terminal complement pathway components in the induction of monocyte TF. We next asked if complement activation facilitates binding of ULICs and promotes subsequent ULIC engagement of FcγRIIA. To examine complement dependent binding of HIT ULICs, we incubated WB with α-C1q Ab prior to addition of KKO ULICs and measured ULIC binding to monocytes and TF expression. As shown in Figure 3, classical pathway inhibition markedly reduced cell-surface IgG (Figure 3A) and monocyte TF expression (Figure 3B). The effects of complement inhibition could not be overcome with increasing amounts of KKO IgG (2-4 fold excess). We observed significant co-localization of complement with KKO ULICs in a cremaster-laser injury model in "HIT mice" and in in situ thrombi formed in uninjured vessels (data not shown). Together, these studies demonstrate an independent role for complement activation in regulating the binding and procoagulant effects of HIT ULICs and identify new non-anticoagulant therapeutic targets that could improve clinical outcomes in this otherwise potentially devastating thrombotic disorder.
Arepally:Novartis: Consultancy; Alexion: Other; Annexon Biosciences: Consultancy, Other; Veralox Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Biokit: Consultancy, Patents & Royalties; Apotex: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.